
Thus if we claim that we accept zero defects and test a very small sample, in this case five samples, there is a high probability that we are accepting defects in the lot without being able to detect them. That would give you an idea of what is done across the board and, then, if you are aq to readjust the workloads, you have some basis for it based on the logs.
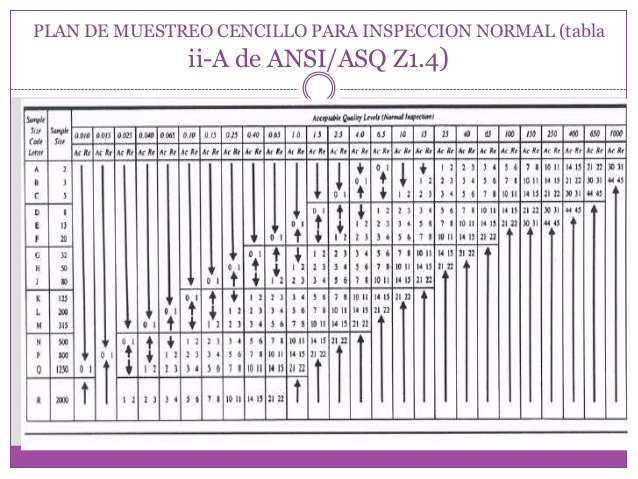
Attributes The FDA recognizes ANSI/ASQ Z as a General consensus standard.Īlthough individual lots with quality as bad as the AQL anzi be accepted with fairly high probability, the designation of an AQL does not suggest that this is necessarily a desirable quality level. This e-standard is a very minor revision of ANSI/ASQ Z (R), also referred to as ANSI/ASQ Z ANSI/ASQ Z Sampling Procedures and Tables for Inspection By.
ANSIASQZSampling Procedures and Tables for Inspection by Attributes- ANSI/ASQ Z Sampling Procedures and Tables for Inspection by.


 0 kommentar(er)
0 kommentar(er)
